Haematopoiesis refers to the developmental process of blood cells. All blood cells are derived from the bone marrow in the adult.
At the end of this module, you should be able to;
- Explain the process of haematopoiesis in the bone marrow
- Describe the components of blood
- Recognise the cellular elements of blood and their respective functions
- Explain the regulation of erythropoiesis by erythropoietin and the function of hypoxia-inducible factors
- Interpret red cell parameters in the context of red cell disorders
Ontogeny of haematopoiesis
The bone marrow is the primary site of haematopoeisis in the adult.
During embryogenesis, early haematopoietic cells develop in the blood islands of the extraembryonic yolk sac. These primitive haematopoietic stem cells (HSC) migrate to the foetal liver, which becomes the main site of haematopoiesis during mid-gestation. Later, the HSCs migrate from the foetal liver to the spleen and marrow. During the third trimester, foetal liver haematopoiesis declines steadily as the spleen and marrow become the major haematopoietic sites.
By the time of birth, the marrow is the major haematopoietic site in humans. During early childhood, haematopoietic cells are found in the marrow of all bones. Haematopoiesis cells in the distal long bones however gradually decline from childhood to late adolescence, and replaced by adipose cells. By adulthood, the haematopoietic marrow is usually limited to the lower skull, vertebrae, shoulder and pelvic girdles, ribs, and sternum.
Although adult haematopoiesis is confined to the marrow, extra medullary haematopoiesis in the liver and spleen may occur under conditions of haematopoietic stress such as in thalassaemia and myeloproliferative neoplasms.
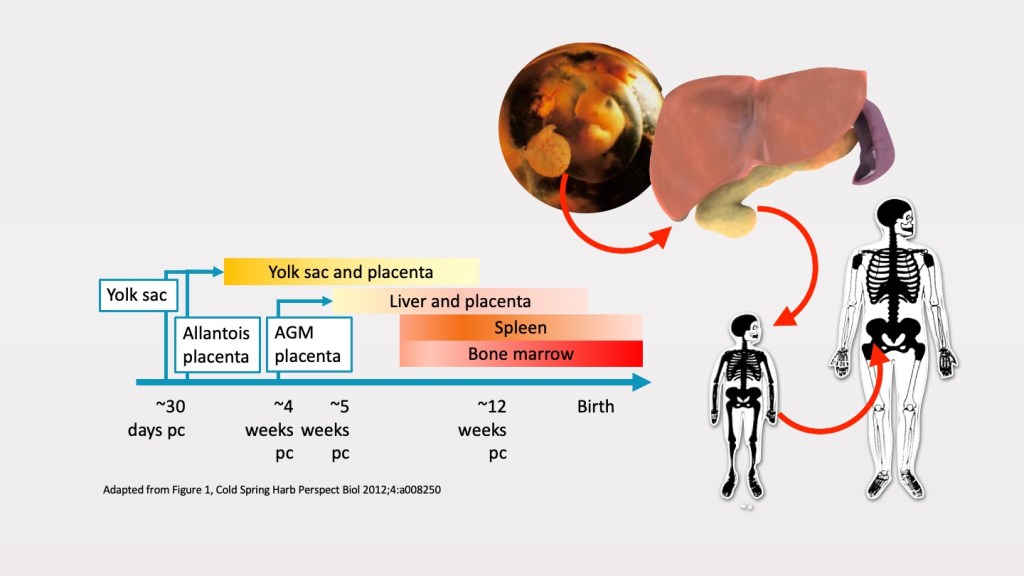
Refer https://cshperspectives.cshlp.org/content/4/12/a008250.full
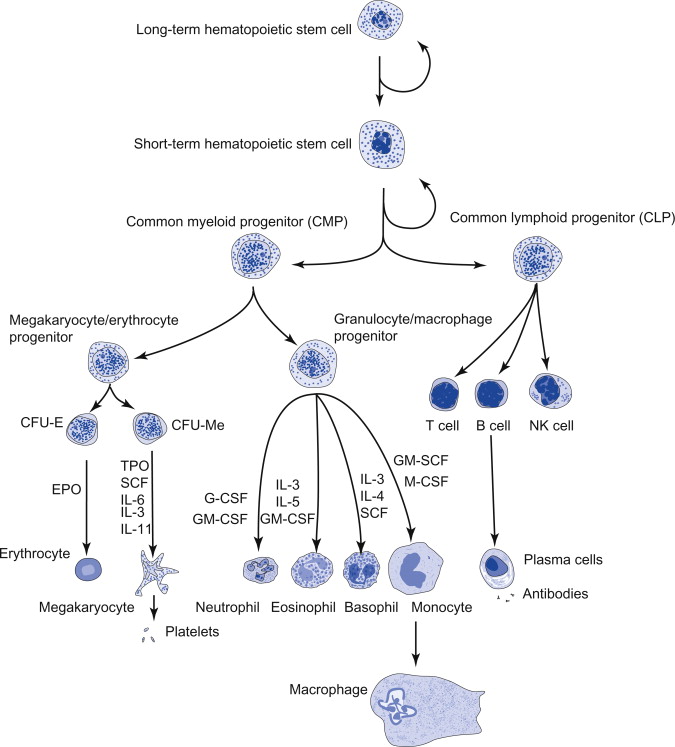
Haematopoiesis
Haematopietic stem cells (HSC)
Haematopietic cells are derived from long-term repopulating haematopoietic stem cells (LT-HSC). These cells are capable of both self-renewal and differentiating capacity. Self renewal enables the cell to make copies of itself and therefore ensures that there is always a pool of HSC which can differentiate and replenish the various haematopoietic cells. HSC express early haematopoietic markers such as CD117 and CD34, and lack lineage specific markers such as CD45.
Multipotent progenitor cells (MPP)
HSC interact with its microenvironmental niche which are composed of bone elements (endosteal-osteoblastic niche) and the bone marrow microvasculature (perivascular-endothelial niche). Depending on signals from the microenvironment and cytokine composition, the LT-HSC can be driven to become multipotent progenitor (MPP) cells. As the HSC differentiate into MPP, the cells lose self renewal capacity while becoming committed to specific haematopoietic lineages.
The two main lineages that MPP differentiate into are the common myeloid progenitor (CMP) and the common lymphoid progenitor (CLP). As the name suggests CMP gives rise to cells of the myeloid lineage while CLP is responsible for cells of lymphoid lineage.
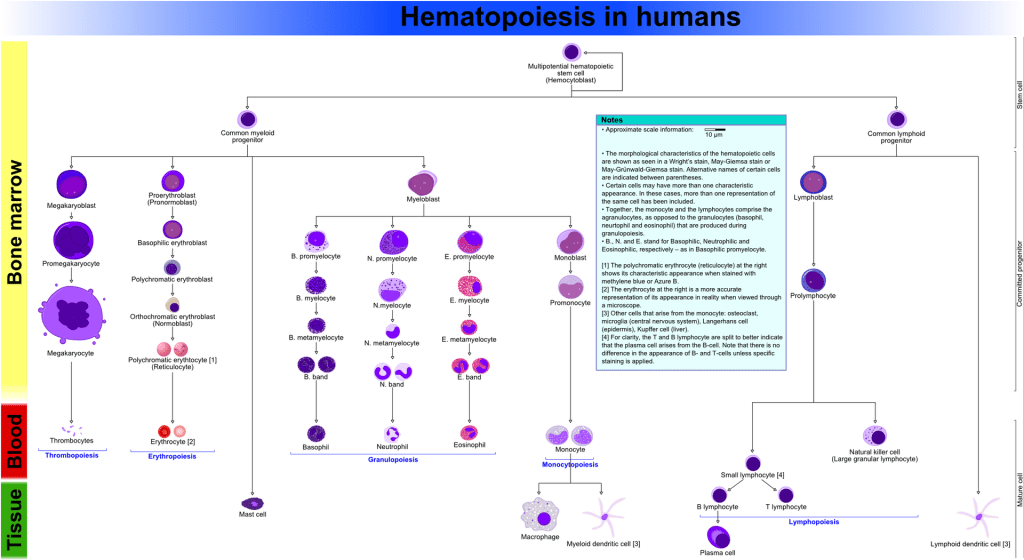
Common myeloid progenitors (CMP)
The common myeloid progenitors (CMP) give rise to cells of the myeloid lineage. CMP can either differentiate into megakaryocyte-erythrocyte progenitors (MEP) or granulocyte-macrophage progenitors (GMP).
Megakayocyte-erythrocyte progenitor (MEP)
The megakaryocyte-erythrocyte progenitors (MEP) undergo a series of maturation steps into megakaryocytes and erythroblast depending on the differentiation signals they receive. Platelets are produced through cytoplasmic budding of the megakaryocytes. The erythroblasts meanwhile differentiate further into normoblasts, which become erythrocytes after extrusion of their nuclei.
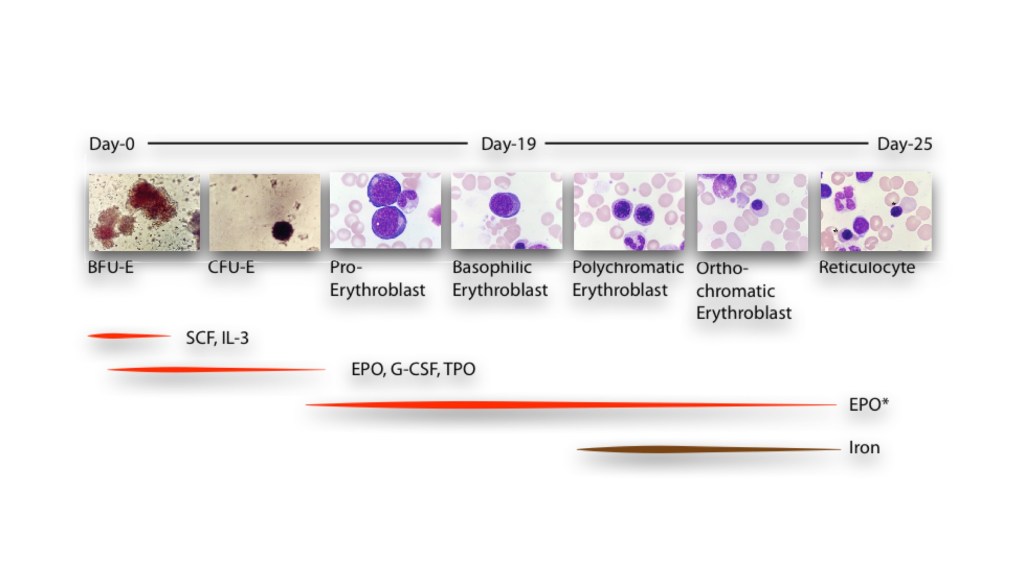
The earliest recognisable stage of erythroid cells on cell culture is the burst-forming-unit-erythrocyte (BFU-E), which appears as a rust-grey collection of cells due to its iron content. These early cells undergo a series of differentiation steps from proerythroblasts into basophilic, polychromatophilic and orthochromatic erythroblasts. These stages are characterised by the gradual change in the shade of cytoplasm on Romanowsky staining from blue (due to high ribonucleic acid content) to red as the cells accumulate haemoglobin with maturation. The nucleus of the erythroblasts also show corresponding maturation and become more dense as they mature until it finally enucleates before being released into circulation as erythrocytes. Erythropoietin and iron are both essential for the development of red cells.
Granulocyte-macrophage progenitors (GMP)
The granulocyte-macrophage progenitors meanwhile differentiate into neutrophils and monocytes. Other cells that are derived from GMP include eosinophils and basophils. Neutrophils and monocytes are capable of phagocytosis and are important cellular mediators of inflammation and innate immunity. Eosinophils and basophils have roles in hypersensitivity reactions. Eosinophils are also important for defence against multicellular organisms such as parasites.