Learning outcomes
- Describe the structure and composition of haemoglobin
- Explain changes in globin chain synthesis from foetal life to adulthood
Regulation of globin synthesis
Haemoglobin is a tetramer composed of four polypeptide globin chains with a central heme component within each of the polypeptide.
The heme component consists of an organic protoporphyrin ring with a central iron ion in the ferrous state (Fe2+). The iron molecule in each of the four heme moiety in a molecule of haemoglobin can bind and unbind oxygen, allowing for oxygen transport in the body.
The globin component of the haemoglobin molecule meanwhile is structured on a pair of globin dimers. The globin chains are transcribed and translated from a group of genes within the 𝜶-globin and 𝜷-globin cluster on chromosome 16 and 11 respectively.
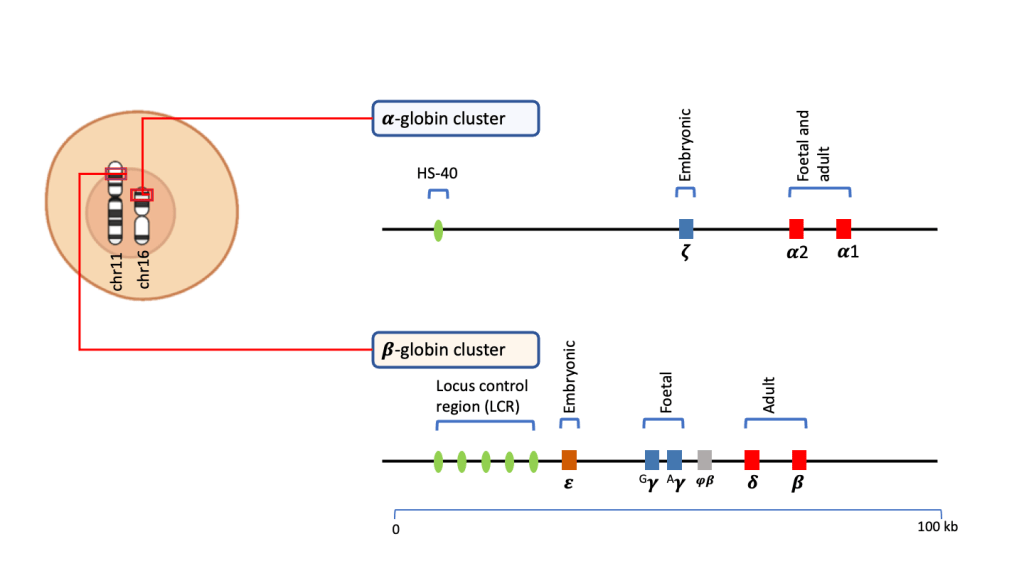
During development from the embryo to adulthood, the genes in either of the clusters are activated and silenced sequentially to form haemoglobin with different paired combinations of globin chains.
- At the embryonic stage, the 𝜻-gene in the the 𝜶-globin cluster is activated together with the 𝜺- and 𝜸-genes within the 𝜷-globin cluster. These globin polypeptides combine to form Hb Gower (𝜻2𝜺2) and Hb Portland (𝜻2𝜸2). The 𝜻-gene and 𝜺-genes aresilenced in later stages of foetal development. Hb Gower and Hb Portland are therefore of no physiological importance thereafter.
- During foetal development, the 𝜶2 and 𝜶1 genes are activated together with the 𝜸-gene, to produce 𝜶-globin and 𝜸-globin chains that combine to form HbF (𝜶2𝜸2). This is the predominant haemoglobin in the foetus. HbF has higher O2 affinity as compared to the adult haemoglobin, HbA (𝜶2𝜷2). This allows the foetus to extract O2 from maternal blood.
- At the later stages of foetal development, the 𝜸-gene is progressively silenced allowing the 𝜷-gene to be active. The production of 𝜷-globin chains lead to the formation of HbA(𝜶2𝜷2), which is the predominant adult haemoglobin.
- At birth, the newborn has both HbF and HbA. HbF levels gradually decline during infancy. After the age of 1-year, the proportion of HbF in total haemoglobin is less than 1%, with about 97-98% composed of HbA. HbA2 (𝜶2𝜹2) is also produced at low levels, comprising about 2% of the haemoglobin.
Beyond the age of 1-year, the main haemoglobin is therefore HbA with only a small component of HbF and HbA2. HbF and HbA2 levels however may be altered due to dysregulated control of the 𝜷-globin cluster, consequent upon mutations within its component genes such as in thalassaemia. Some acquired conditions causing abnormal red cell maturation as seen in some forms of leukemias may also cause a slight elevation in HbF levels.