Learning outcomes
- Explain the role of glucose-6-phosphate dehydrogenase (G6PD) enzyme in protection of red cells from oxidative damage
- Describe the genetics, clinical features and laboratory investigation of G6PD deficiency
- List factors that should be avoided in patients with G6PD deficiency
Red cell metabolism
Red cells have limited metabolic activity as they do not have nuclei or cytoplasmic organelles including mitochondria. Nonetheless, red cells need to accomplish many tasks, including maintaining their cellular shape and avoiding damage from oxidants, all which require an energy source.
Glucose is the major energy source for the red blood cell. Red cells cannot depend on aerobic glycolysis, as in the Kreb’s cycle, to extract energy from glucose, because they lack the oxidative enzymes present in mitochondria. They therefore use the Embden-Meyerhof pathway (EMP) to anaerobically process glucose into adenosine triphosphate (ATP).
In addition to generating ATP to maintain red cell function, red cells also need to maintain a store of reduced nicotinamide adenine dinucleotide phosphate (NADPH). NADPH is required to convert oxidised glutathione to reduced glutathione which acts as the major red blood cell antioxidant. Failure in generation of NADPH through the pentose-phosphate pathway (also known as hexose monophosphate shunt (HMP) exposes red cells to oxidant damage.
Enzyme defects have been described with many of the metabolic processes that occur in red cell. Two of the most common red cell enzyme defects are;
- Pyruvate kinase (PK) deficiency which is mainly found in Western population
- Glucose-6-phosphate dehydrogenase (G6PD) deficiency found in Asia, Africa and the Mediterranean.
Glucose-6-phosphate dehydrogenase (G6PD) deficiency
G6PD deficiency is the most common inborn error of metabolism worldwide, affecting over 400 million individuals worldwide. It is common in malaria-endemic areas, including Malaysia, and has a protective role in Plasmodium falciparum infection.
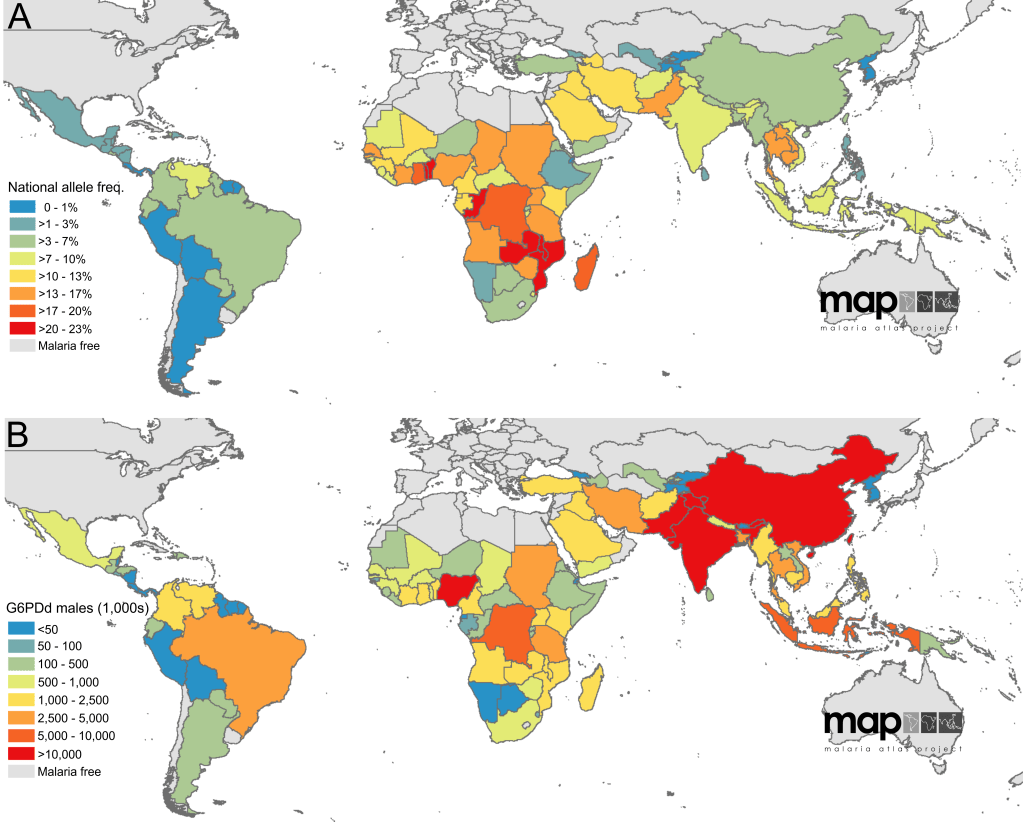
Inheritance
G6PD deficiency is a X-linked recessive condition. Hemizygous males and homozygous females express the condition, while heterozygous females are usually unaffected or show mild degrees of deficiency.
Pathophysiology
The spectrum of G6PD mutations is wide and heterogenous worldwide as is the severity of the disease. G6PD deficient individuals have normal levels of G6PD enzyme in reticulocytes and young red cells. Levels however decline rapidly with red cell ageing as the defective enzyme produced by the mutated gene are labile and have shortened half life.
The primary role of G6PD in the pentose-phosphate pathway (also known as the hexose-monophosphate shunt) is the generation of NADPH which is required for reduction of oxidised glutathione.
Oxidants are continuously generated in red cells. Formation of the reactive oxygen species (ROS), H2O2, can occur spontaneously from O2 or is generated via superoxide dismutase (SOD) action on superoxide anion produced as a result of O2 oxidation of the ferrous iron (Fe2+) to ferric iron (Fe3+) in hemoglobin. In addition, various insults such as infection, drugs, chemical intermediaries and diet also contribute to ROS generation.
Reduced glutathione (GSH) is a natural anti-oxidant that neutralises ROS through the donation of a hydrogen atom. GSH is oxidised in the process to oxidised glutathione (GSSG) by glutathione peroxidase.
2 GSH + H2O2 → GSSG + 2 ROH

The oxidised glutathione, GSSG must be reduced back to GSH to regenerate the anti-oxidant GSH, failing which red cells are susceptible to oxidant damage. As emphasised earlier, red cells are devoid of protein synthetic activity and cannot form new glutathione molecules. Whatever GSH is required must be regenerated from GSSG.
The hydrogen atom required for GSSG reduction to GSH is supplied by NADPH, in which glutathione reductase reduces GSSG with the following reaction:
NADPH + GSSG + H2O → 2 GSH + NADP+ + OH−
The NADPH is generated from the rate limiting step of the pentose-phosphate pathway that is catalysed by G6PD.
Glucose-6-phosphate + NADP+ → 6-Phosphogluconolactone + NADPH + H+
Deficiency of G6PD activity due to a defective enzyme hence disrupts NADPH generation, which in turn prevents regeneration of GSH. The lack of anti-oxidant GSH activity contributes to uncontrolled oxidative damage to red cells, especially when subjected to oxidative stress.
Clinical features
The clinical presentation is variable depending on the type of mutation in the patient. General presentations may include;
- Acute intravascular haemolysis following exposure to oxidants. Ingestion or exposure to the oxidant may precipitate an attack of acute intravascular haemolysis with rapid drop of haemoglobin, onset of jaundice and passing dark urine. Recognised oxidants include
- antimicrobials – sulphonamides, dapsone, nitrofurantoin
- antimalarials – primaquine, chloroquine, quinine, mepacrine
- analgesics – aspirin
- napthalene – moth balls
- rasburicase
- ingestion of broad beans (lava beans, kacang parang)
- Haemolysis during intercurrent acute illnesses such as bacterial infections, diabetic ketoacidosis and viral hepatitis
- Neonatal jaundice. Newborns may develop haemolysis early after birth and within the first three weeks of life. The severe hyperbilirubinaemia can lead to kernicterus, in which bilirubin accumulates in the brain, causing long-term neurological damage.
- Chronic non-spherocytic haemolytic anaemia

Laboratory features
- Screening and identification. All newborns in Malaysia are screened for G6PD deficiency, so that appropriate advice can be provided to the parents and the newborn observed for neonatal jaundice.
- During acute haemolytic episodes, the patients may show;
- Hyperbilirubinaemia
- Increase lactate dehydrogenase (due to breakdown of red cells)
- Reduced haptoglobin
- Characteristic red cell changes on the peripheral blood film – reticulocytosis, bite cells
- Haemoglobinuria and haemoglobinaemia
- Urobilirubinuria
- Positive G6PD test (the test may sometime be false negative if there is high reticulocytosis, as reticulocytes contain normal amounts of G6PD)
- Patients with chronic haemolysis would show biochemical features that is indistinguishable from other causes of haemolytic anaemia. The peripheral blood film is usually unremarkable except for reticulocytosis. G6PD activity would however be low.
Treatment
Screening and appropriate counselling to ensure avoidance of precipitating oxidants is important. Newborn screening is advocated in high incidence areas.
Management of acute haemolytic episodes is supportive. Blood transfusion may be necessary sometimes. Neonatal jaundice should be treated aggressively with phototherapy and red cell exchange if severe hyperbilirubinaemia. Patients with chronic haemolysis should receive folate supplementation.
